How a Star Turns Simple Atoms Into Light, Heat, and Us
In the time it takes to read this sentence, the Sun has poured out more energy than humanity uses in roughly 600,000 years. All that power streams away as heat and radiation—light that warms our skin, drives the weather, and fuels every leaf on Earth.
But where does that staggering energy come from? What is the Sun burning, if it’s not coal, oil, or anything we’d recognize as fuel? And how does it keep shining so steadily that life on Earth has thrived for billions of years?
To answer that, we need to dive into the crowded, furious heart of a star, where atoms behave in ways they never do on Earth.
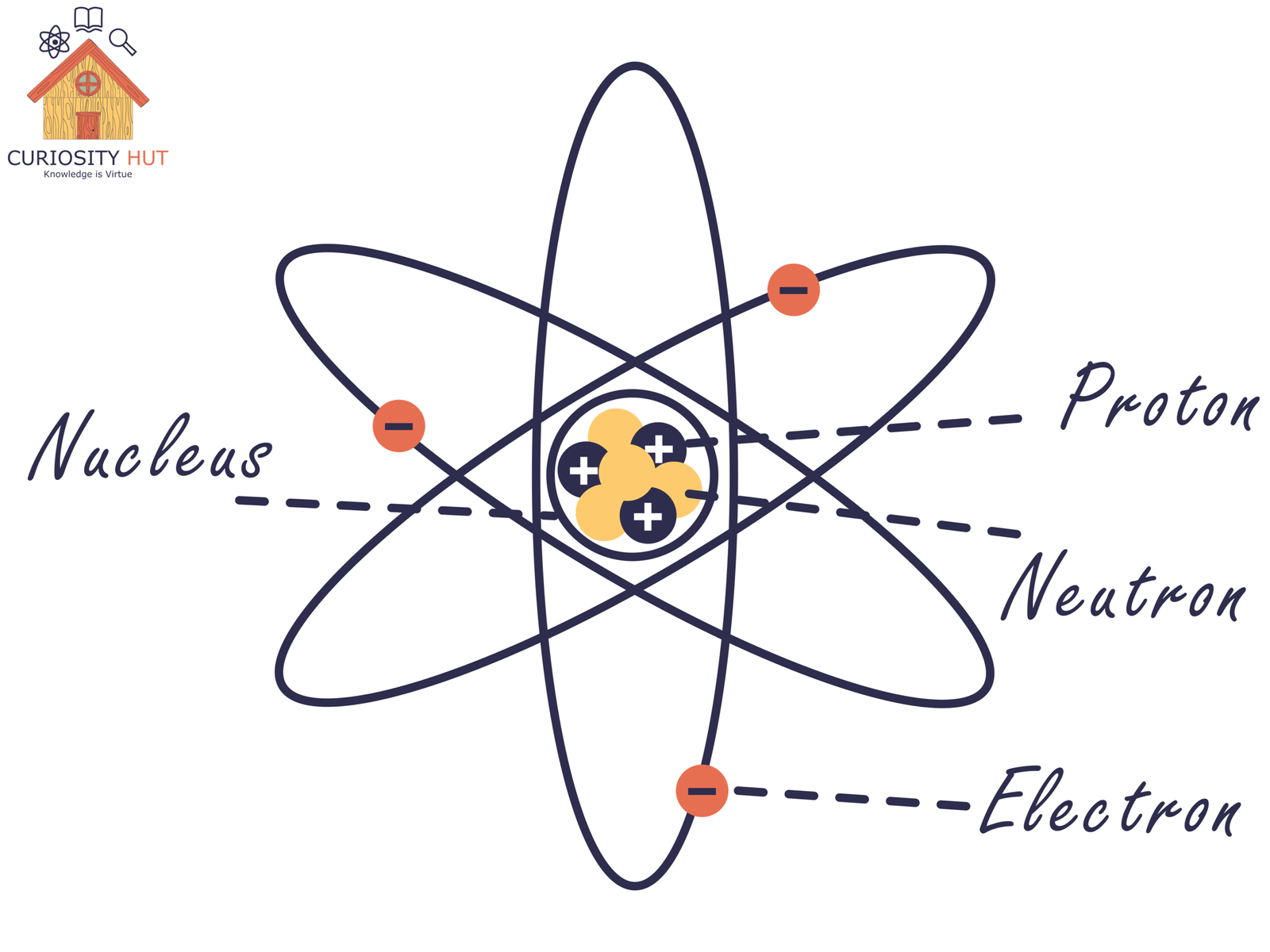
Everything you can touch, taste, or trip over is made of atoms. Picture an atom as a tiny solar system: a dense central nucleus surrounded by a whirling cloud of electrons. The electrons set the size, but the nucleus carries almost all the weight.
The nucleus itself is a tight bundle of protons and neutrons. Protons are positively charged; neutrons carry no charge. They’re packed so closely together that the forces inside the nucleus are unlike anything we experience in daily life. This force is so strong it locks away an enormous amount of energy—energy that only releases when the nucleus is rearranged.
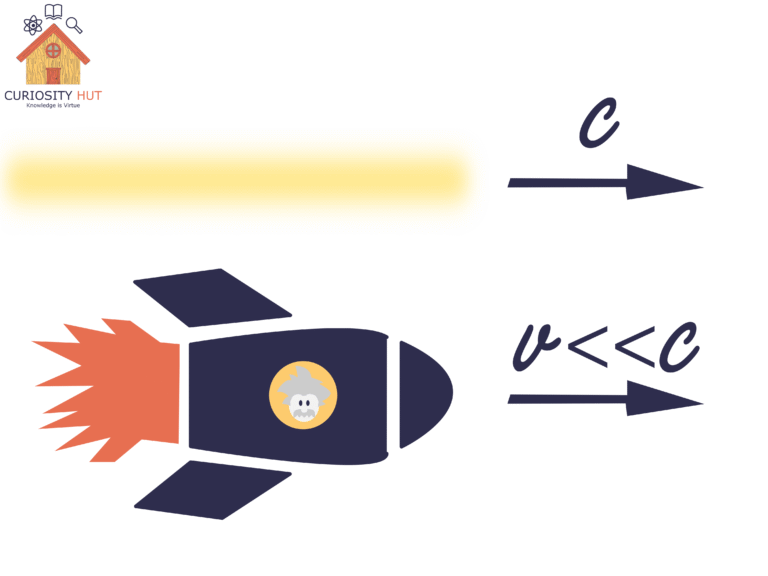
This is where the famous equation comes in. E = mc² shows that mass and energy are two sides of the same coin. The c²—the speed of light squared—is huge, so even a tiny loss of mass produces a vast amount of energy. Turn the mass of a paperclip entirely into energy, and it would outpower some countries for a year. That’s the scale we’re talking about in the Sun.
Fusion taps into this mass–energy link. When light nuclei merge into something heavier, their combined mass ends up slightly smaller than the sum of the ingredients. That missing mass doesn’t vanish; it transforms into energy. In the Sun’s core, this happens trillions of times each second, producing a radiance that lights and warms an entire solar system.
Subscribe to my Free Newsletter
Sign up for blog updates, science stories, riddles, and occasional musings. No algorithms—just me, writing to you.
Where the Heat Comes From: Squeezing Hydrogen Until It Changes
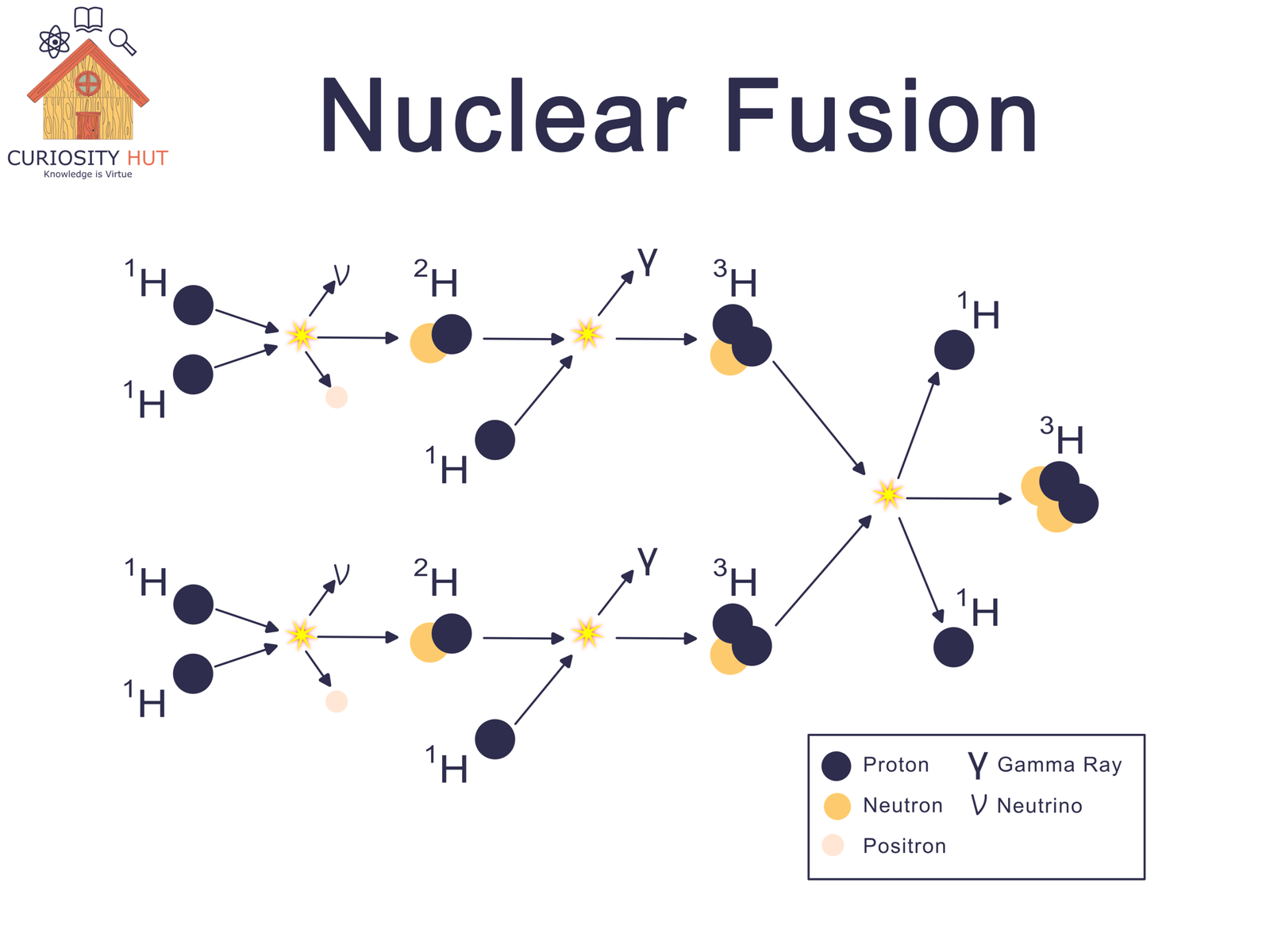
At the Sun’s center, hydrogen exists as bare nuclei—protons racing through a plasma hotter than 15 million °C. Gravity packs them so tightly that pressure becomes overwhelming. Under these conditions, protons collide with enough force to overcome their natural repulsion.
Fusion begins with that unlikely meeting. Two protons crash together; one transforms into a neutron, creating a heavier form of hydrogen. This new nucleus then fuses with another proton, forming helium’s building blocks. Step by step, four original protons become one helium nucleus.
Each step trims a tiny amount of mass from the ingredients. That lost mass becomes energy, exactly as E = mc² predicts. Multiply this by billions of trillions of reactions every second, and you get the steady flood of heat and light that streams from the Sun.
Helium holds its particles together more tightly than hydrogen. That tighter grip is called binding energy—the energy that locks a nucleus in place. When light nuclei fuse, the new nucleus needs less mass to store the same amount of “nuclear glue,” so the extra mass is freed as energy.
Fusion works best for the lightest elements. As nuclei get heavier, binding energy per particle increases until it peaks at iron. Fusing anything heavier than iron costs energy instead of releasing it. This natural limit shapes every star’s life, including our Sun’s.
How the Sun Keeps Itself Stable
Fusion happens only in the Sun’s core, where pressure and temperature are extreme enough to force nuclei together. If fusion speeds up, the core heats and expands slightly, lowering pressure and slowing fusion. If fusion slows, the core contracts, pressure rises, and reactions pick up again.
This self-regulating tug-of-war keeps the Sun steady. Every second, it converts about 620 million tons of hydrogen into helium, releasing just enough energy to balance gravity’s inward pull. The result is a star that burns smoothly for billions of years.
How Stars Build the Rest of the Elements
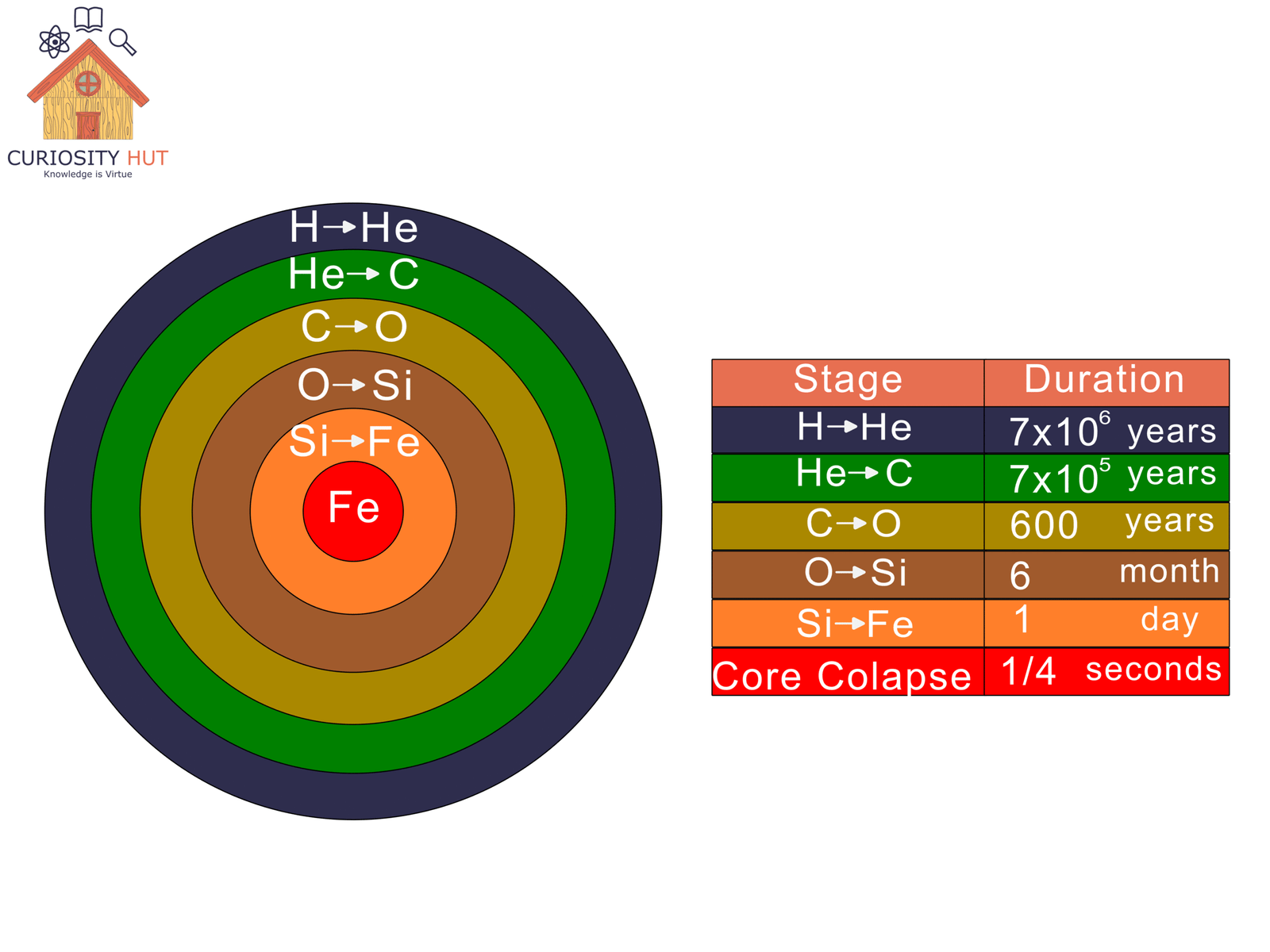
The Sun fuses hydrogen into helium, but heavier elements need far more extreme conditions. Massive stars—much larger than ours—reach higher temperatures, fusing helium into carbon, carbon into oxygen, and so on, climbing the periodic table step by step until iron forms.
Iron is the dead end. Once a core fills with it, fusion can no longer generate energy. The star collapses under its own weight and rebounds in a supernova. That explosion forges the rest of the elements—gold, iodine, uranium, calcium, iron—scattering them across space.
Everything heavier than hydrogen and helium was made this way. Planets, oceans, and people are built from atoms once blasted into space by dying stars.
Why We Can’t Yet Recreate Stellar Fusion on Earth
The Sun has one advantage we can’t match: sheer gravity. Its entire mass squeezes the core so hard that fusion runs steadily without flying apart. On Earth, we must fake that pressure using magnetic fields or powerful lasers, heating hydrogen fuel to more than 100 million °C—hotter than the Sun’s core—just to give nuclei a chance to fuse.
The challenge is containment. At those temperatures, matter becomes plasma, a substance that slips through anything solid. Magnetic “bottles” try to hold it in place, but the plasma wriggles, swirls, and leaks energy faster than we can sustain the reaction. Experiments produce brief bursts of fusion energy, but none have held it long or efficiently enough to power a grid.
We’re getting closer, but building a stable star on a small planet remains a far tougher engineering problem than nature ever had to solve.
A Universe in Every Atom
The Sun may seem ordinary, but it is a furnace where hydrogen is transformed into helium, radiating energy that warms our planet every second. Beyond the Sun, massive stars continue the process, forging the elements that make up everything we see—and everything we are. Every atom heavier than hydrogen in your body was once part of a star, scattered across space in a cosmic explosion. Humnas are just stardust with an electric Jello in our heads.
When you look at the sky, you’re seeing history: the story of matter, energy, and life itself. In a very real sense, we are stardust, shaped by fusion, bound by gravity, and connected to the universe in ways we are only beginning to understand.
Subscribe to my Free Newsletter
Sign up for blog updates, science stories, riddles, and occasional musings. No algorithms—just me, writing to you.